2020 官网升级中!现在您访问官网的浏览器设备分辨率宽度低于1280px请使用高分辨率宽度访问。
Time:2022-01-12
Click:22
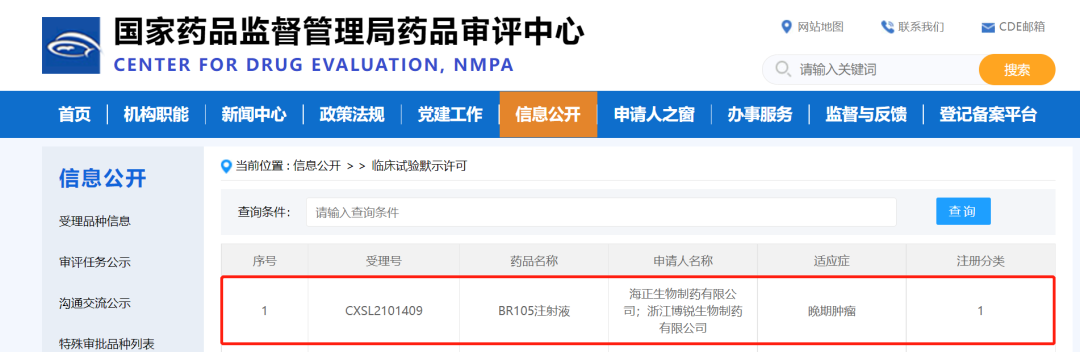
Taizhou, Zhejiang, China, Jan. 12, 2022 -- BioRay Pharmaceutical Co., Ltd. (hereinafter referred to as BioRay) announced that the National Medical Products Administration (NMPA) has approved the Investigational New Drug (IND) application for the self-developed Category 1 innovative drug BR105 for oncology treatment. As a novel agent for anti-tumor immunotherapy, BR105 is a SIRPα-targeting humanized monoclonal antibody that was independently developed by BioRay, which can recognize common variants of signal-regulated protein α (SIRPα), blocks the binding of SIRPα to CD47, disengage the “don’t eat me” signal and activate macrophages to promote phagocytosis of tumor cells.
SIRPα is a transmembrane protein expressed on the surface of myeloid cells such as macrophages, monocytes, dendritic cells, and granulocytes. CD47 is the major ligand of SIRPα, which is widely expressed on the surfaces of normal cells. As one of the important mechanisms of tumor evasion from immune surveillance, CD47/SIRPα signaling is closely related to tumor progression and metastasis. The SIRPα-CD47 interaction transmits a ‘do not eat me’ signal for immune evasion and prevents the phagocytosis of tumor cells by macrophages. Therefore, targeting CD47/SIRPα has become a promising strategy to promotes anti-tumor immunotherapy. Clinical studies suggest that blockage of the CD47–SIRPα signaling pathway has shown the positive efficacy results in acute myeloid leukemia, lymphoma, head and neck squamous cell carcinoma, gastric cancer, and other tumors.
SIRPα has the potential to be used in combination with T-cell immune checkpoint inhibitors such as anti-PD-1 antibodies and anti-PD-L1 antibodies, which is expected to solve the problems of drug resistance and non-response faced by PD-1/PD-L1 inhibitors in clinical practice. BR105 inhibits the CD47/SIRPα signaling pathway by targeting SIRPα, which avoids the hematologic toxicity by targeting CD47 and is superior in safety. Targeting SIRPα could activates innate immune responses by activating myeloid cells. On the other hand, BR105 plays a synergistic role with antibodies targeting tumor-associated (TAA) antigens to further enhance the tumor-killing effect of TAA antibody-mediated immune cells.
In preclinical studies, BR105 was able to bind to different variants of SIRPα and block the SIRPα-CD47 interaction. The in vitro and in vivo efficacy of BR105 showed that it could effectively relieve CD47/SIRPα-mediated phagocytosis inhibition signal and promote anti-tumor immune response; moreover, BR105 did not affect the T cell activation signal involved in SIRPγ. Meanwhile, in toxicological studies, BR105 did not cause hematological toxicity and showed excellent safety.
CD47/SIRPα is considered one of the most prospective targets for tumor immunotherapy after PD-1/PD-L1. There is no monoclonal antibody targeting SIRPα in the worldwide market up to now. Dr. Haibin Wang, Chief Executive Officer of BioRay, said, "The approval of BR105 injection in clinical trials will further enrich BioRay's product pipeline and demonstrates BioRay's determination to provide a comprehensive and diverse product portfolio for oncology patients. We are in the process of laying out the next-generation targets, and our research and development programs in the areas of popular tumor immune targets and autoimmunity are progressing steadily. We will make every effort to advance the clinical trials of BR105 and expect that it will be approved for marketing as soon as possible to benefit the majority of oncology patients.

TO TOP